By Vidja Rajan, Columnist, The Times
 Easter, with its egg hunts and chocolate eggs, is past. With the current school closures and social distancing, the kids are bouncing off the walls already, and don’t need any further stimulation. It’s time to get rid of the chocolate. But the reliable excess food disposal unit, aka Man’s Best Friend, is forbidden from gormandizing Godivas.
Easter, with its egg hunts and chocolate eggs, is past. With the current school closures and social distancing, the kids are bouncing off the walls already, and don’t need any further stimulation. It’s time to get rid of the chocolate. But the reliable excess food disposal unit, aka Man’s Best Friend, is forbidden from gormandizing Godivas.
Why so?
In this installment of Inner Nature, I explore why other animals don’t share a similar metabolism of food as humans. For example, a vulture will quite happily devour putrefying meat that would cause a human food poisoning. And Rover would die if he gets too much chocolate. What is it about chocolate that nourishes us but kills dogs?
Chocolate is one of the luxuries of the food realm. Humans ingest stupefying quantities of chocolate. Chocolate commands a global market that is twice the value of the global coffee market, and equivalent to the global diamond market. Maybe that is why there is a romantic equivalence between chocolate and diamonds!
But chocolate is not for everyone or, rather, every species. An ounce of milk chocolate, or 0.1 oz of unsweetened baking chocolate, can be fatal to your family dog. Chocolate contains the alkaloids theobromine and caffeine, the biogenic amines tyramine, tryptamine, serotonin, and 2-phenylethylamine, and brain cannabinoids [1]. For humans, these are feel-good chemicals and stimulants that bind to brain, heart, and lung receptors. Serotonin is a reward neurotransmitter, and cannabinoids are addictive, causing chocoholics to crave the next “hit”. Caffeine and theobromine are stimulants, causing alertness and increasing the pulse rate in both people and dogs. The crucial difference is that humans can metabolize these molecules to break them down. Dogs lack CYP1A2, which is responsible for breaking down caffeine and theobromine in the human liver. The absence of this enzyme allows these molecules to persist in the bloodstream much longer and to build up to toxic concentrations quickly.
Caffeine and theobromine are derived from a class of molecules called purines, and so are adenine (A) and guanine (G), which are components of DNA. Adenine performs different functions depending on slight differences in the carrier to which it is attached. In DNA, adenine is part of the genetic code. It is found in life’s central energy molecule, ATP. It carries electrons as NADH. It is a catalyst as coenzyme A. Cyclized, it is a messenger molecule called cyclic AMP. Take a look at Figure 1 – notice that adenine and caffeine have the same basic structure. But here’s the kicker – although they are chemically similar, caffeine and adenine have diametrically different actions. Adenine binding causes generally slowing down and reduction of cellular activity: depressed neuron activity; slowed cardiac contractions; relaxed skeletal muscles. Caffeine stimulates alertness and activity. Physiologically speaking, caffeine is an adenine antagonist, meaning it opposes the actions of adenine [2]. Blocking adenine from binding causes neurons to get excited, cardiac contractions to increase, and muscles to contract.This is fine if you are a human because your enzymes will break down caffeine but, since Rover cannot, he will have seizures, tachycardia, and muscle spasms. Not only can Rover not tolerate the caffeine in chocolate, that espresso will do him no good either.
Purines were characterized and named by the German chemist Emil Fischer – purine is an abbreviation of “pure urine” – because uric acid is a constituent of urine. If blood levels of uric acid increase too much it can crystallize as stones in the kidney or in the joints to cause gout. In his stellar career, Emil Fischer also established the structures of indole-based chemical dyes and the stereoisomers of glucose, synthesized barbituate sedatives, isolated amino acids and used them to synthesize peptides, proposed the Lock-and-Key hypothesis for enzyme action, and described structures for chemical compounds (today called Fischer Projections). He was a one-man whirlwind of discovery, and was awarded the second-ever Nobel Prize in Chemistry in 1902 for his work on purines and sugars.
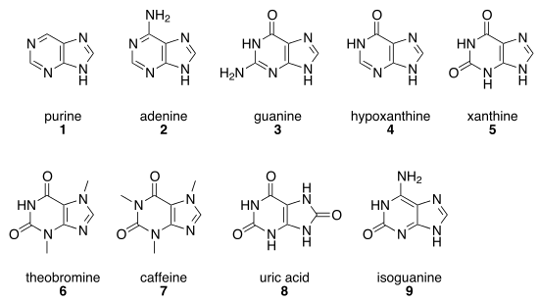
Figure 1: Notable purines. All purines share a heterocyclic ring fused to an imidazole ring. Derivations of the purine base affect the molecule’s biological action. Public domain figure reproduced from: https://en.wikipedia.org/wiki/Purine.
So. Coffee and chocolate: good for humans, bad for dogs. What about invertebrates, specifically bees?
Caffeine is generally regarded as a chemical deterrent against herbivory since it is bitter and unpalatable in addition to its psychoactive effects. However, coffee and citrus plants include small amounts of caffeine in nectar. This is puzzling, because nectar is a reward to pollinators, and the inclusion of caffeine would seem to contradict the purpose of the reward. Further examination showed that caffeine works in bees exactly as it does in dogs and humans. It blocks adenine receptors causing activation of neurons in the bee’s brain. This causes bees to remember the source of the caffeine-laced nectar better than available controls [3]. But, significantly, concentrations of caffeine in nectar are lower than the bitter taste-sensing mechanism of the bees, so they receive the memory jolt without the unpalatable taste. As far as the plant is concerned, higher concentrations in leaves could still deter herbivory, but lower concentrations in nectar would encourage pollination.
Win-win.
Note to chemistry purists: I have conflated adenine and adenosine in the above explanation to maintain comprehension. Adenosine is adenine attached to a ribose sugar.
- Di Tomaso, E., M. Beltramo, and D. Piomelli, Brain cannabinoids in chocolate. Nature, 1996. 382(6593): p. 677.
- Franco, R., A. Oñatibia-Astibia, and E. Martínez-Pinilla, Health benefits of methylxanthines in cacao and chocolate. Nutrients, 2013. 5(10): p. 4159-4173.
- Wright, G., et al., Caffeine in floral nectar enhances a pollinator’s memory of reward. Science, 2013. 339(6124): p. 1202-1204.






